Supplies available from ATCC
- 3T3-L1 Mouse Embryonic Fibroblasts, ATCC CL-173
- Dulbecco’s Modified Eagle’s Medium (DMEM), ATCC 30-2002
- Bovine Calf Serum, ATCC 30-2030
- Fetal Bovine Serum (FBS), ATCC 30-2020
Additional supplies
- Insulin (bovine), Sigma Aldrich I0516
- Dexamethasone, G Biosciences API-04
- IBMX (Sigma Aldrich I5879)
Media formulations used throughout
| Medium | |
|---|---|
| Pre-adipocyte Expansion Medium | 90% DMEM 10% Bovine Calf Serum |
| Differentiation Medium | 90% DMEM 10% FBS 1.0 µM Dexamethasone 0.5 mM IBMX 1.0 µg/mL Insulin |
| Adipocyte Maintenance Medium | 90% DMEM 10% FBS 1.0 µg/mL Insulin |
| Pre-adipocyte Expansion Procedure | Maintain the cells in the Pre-adipocyte Expansion Medium according to instructions for handling provided in the 3T3-L1 product sheet. Seed the cells at about 3000 cells per cm2 and never allow the cells to become confluent. Subculture 3T3-L1 cells before cultures reach 70% confluence (Figure 1). |
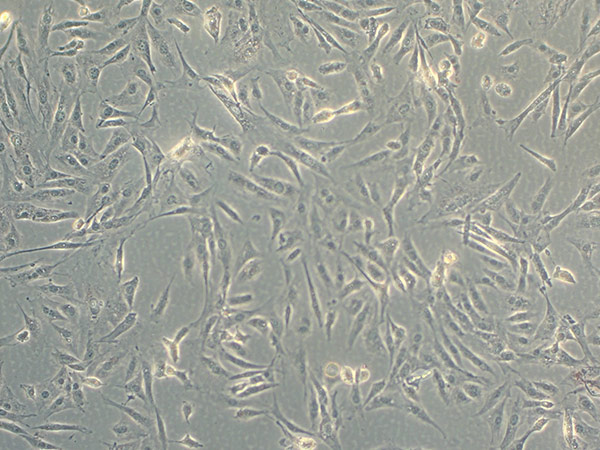
Figure 1. Light microscopy of subconfluent 3T3-L1 cells.
Differentiation procedure
- When the cells are 70-80% confluent, harvest the cells by trypsinization according to the instructions related to subculture.
- Seed the cells in the desired culture vessel in the Pre-adipocyte Expansion Medium.
| Culture vessel | Cell density |
|---|---|
| 6 well plate | 8 × 104 cells/well |
| 24 well plate | 2 × 104 cells/well |
| 96 well plate | 2 × 103 cells/well |
- Let the cells grow for 48 hours, or until the culture reaches 100% confluence (Figure 2 & Figure 3). Feed the cells with the Pre-adipocyte Expansion Medium after 48 hours.
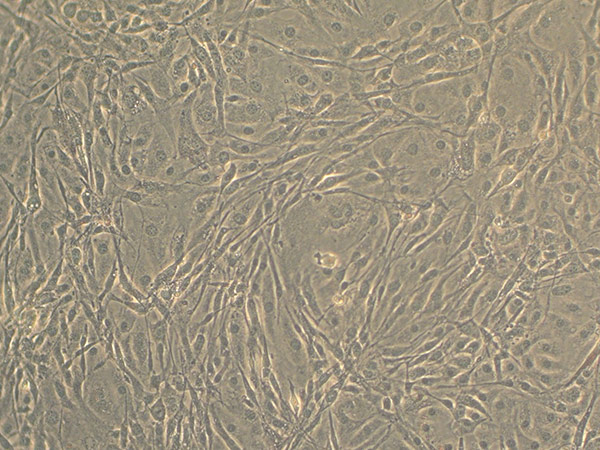
Figure 2. Light microscopy of confluent 3T3-L1 cells.
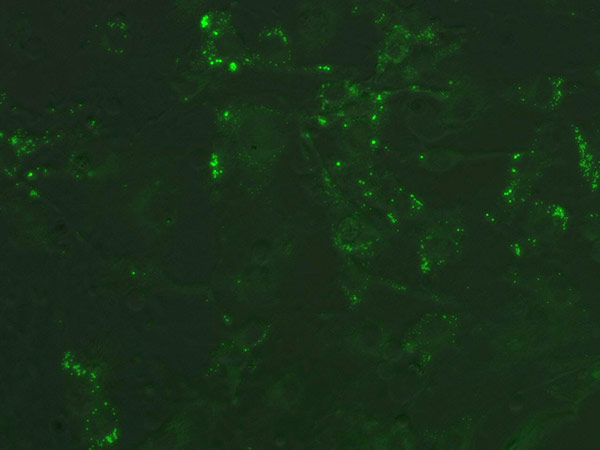
Figure 3. Confluent 3T3-L1 cells stained with Nile Red prior to induction.
- Incubate the cells as a confluent culture for another 48 hours.
- Remove the growth medium from each well and add an identical volume of Differentiation Medium.
- Incubate in Differentiation Medium for 48 hours.
- After 48 hours, replace the Differentiation Medium with Adipocyte Maintenance Medium.
NOTE: At this stage, the cells detach easily from the tissue culture vessel. Gentle pipetting/handling is recommended from this point forward. - Replace the Adipocyte Maintenance Medium every 48 to 72 hours.
- The cells should be fully differentiated between 7 to 15 days after induction, as evidenced by observation of lipid droplet formation (Figure 4 & Figure 5).
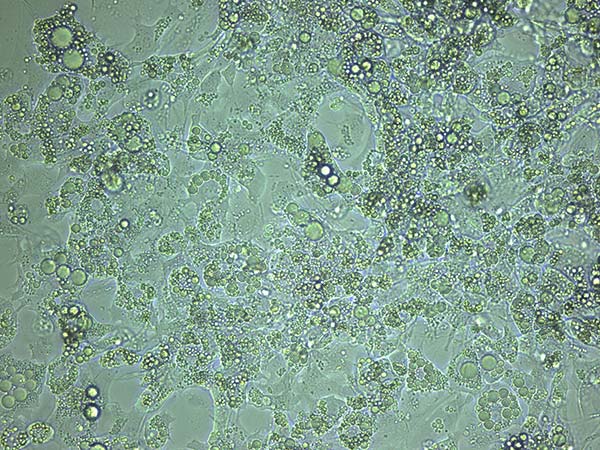
Figure 4. Light microscopy of differentiated 3T3-L1 cells.
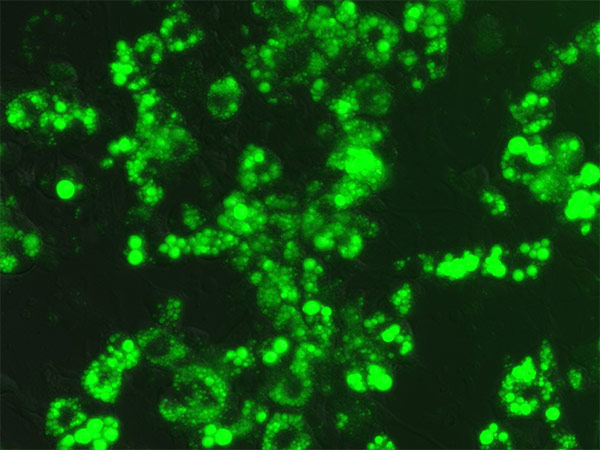
Figure 5. Differentiated 3T3-L1 cells stained with Nile Red.
Troubleshooting
Why do the cells peel off the substrate when I add the Differentiation Medium?
Possible cause: pH and osmolality of the Differentiation Medium fall outside of the normal physiological range.
Solution: Check pH and osmolality of the Differentiation Medium prior to use. pH should fall between 7.0 and 7.4, and osmolality should falls within 270 to 365 mOsm/kg.
Possible cause: Cells were left for too long without a liquid layer.
Solution: Take great care to immediately add Differentiation Medium to the well once the Pre-adipocyte Expansion Medium is removed.
Why do the cells detach after I add the Adipocyte Maintenance Medium?
Possible cause: The differentiated cells were handled too harshly and detached.
Solution: The cells easily detach once differentiation occurs. Take care when adding or removing medium. If the cells detach, add fresh medium to spent cultures and incubate for 48 hours.
Possible cause: Cells were left for too long without a liquid layer. pH and osmolality of the Adipocyte Maintenance Medium fall outside of the normal physiological range.
Solution: Check pH and osmolality of the Adipocyte Maintenance Medium prior to use. pH should fall between 7.0 and 7.4, and osmolality should fall within 270 to 365 mOsm/kg.
Possible cause: Cells were left for too long without a liquid layer.
Solution: Take great care to immediately add Adipocyte Maintenance Medium to the well once the Differentiation Medium is removed.
Why haven’t the cells formed lipid droplets after 2 weeks?
Possible cause: The wrong reagents were used (eg human insulin was substituted for bovine insulin).
Solution: If using an alternative supplier, be sure to check the quality, purity and/or characterization (eg cell-culture-tested) of the material prior to use.
Possible cause: The differentiation reagents were added to the medium in the wrong amounts.
Solution: Over- or under-supplementing the Differentiation Medium could keep the cells from forming lipid droplets; it may also, in the case of over-supplementing, cause irreversible harm to the cells.
Possible cause: The cells were not confluent prior to the addition of the Differentiation Medium.
Solution: Be sure to allow the culture to reach 100% confluence prior to changing to the Differentiation Medium (ie at confluence, the cells are already primed to differentiate).
Possible cause: The cells may not have been exposed to the Differentiation Medium long enough.
Solution: Once the Differentiation Medium is added, be sure to expose the cells for a full 48 hours.
Possible cause: Passage effect.
Solution: The cells may have been over-passaged or kept too dense during routine subculture.
Download a PDF of this technical document
Download NowReferences
- Neal JW, Clipstone NA. Calcineurin mediates the calcium-dependent inhibition of adipocyte differentiation in 3T3-L1 cells. J Biol Chem 277(51):49776-49781, 2002.
- Barnes DM, Hanlon PR, Kircher EA. Effects of inorganic HgCl2 on adipgenesis. Toxicol Sci 75:368-377, 2003.
- Madsen L, et al. Adipocyte differentiation of 3T3-L1 preadipocytes is dependent on lipoxygenase activity during the initial stages of the differentiation process. J Biochem 375:539-549, 2003.
- Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev 78(3):783-809, 1998.
- Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell 3:127-133, 1974.
- Green H. Triglyceride-accumulating clonal cell line. U.S. Pat. 4,003,789 dated Jan. 18, 1977.
