Comparison of Label Free Imaging Approaches and Destructive In Vitro Cell-based Assays for the Assessment of Growth and Viability of Primary Patient-derived Organoid Cancer Models
ASCB/EMBO 2019
Washington, DC, United States
December 07, 2019Abstract
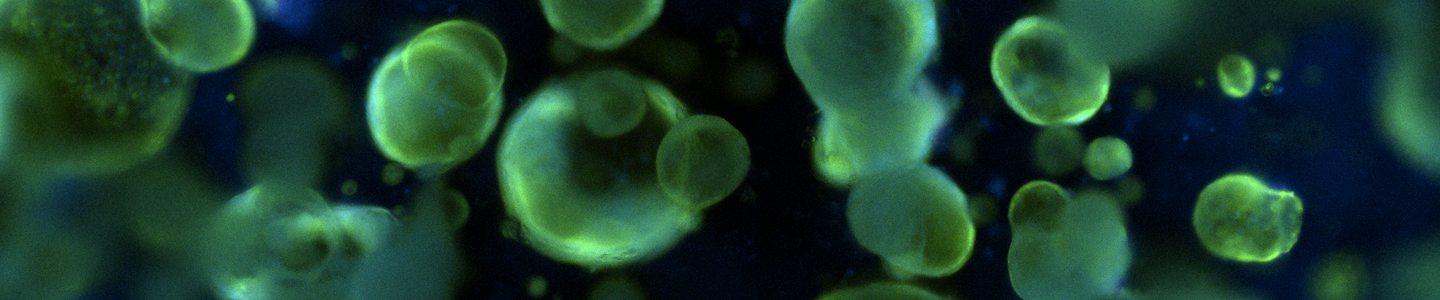
One major challenge in cancer biology is the lack of physiologically relevant models that have long-term expansion potential in vitro but still maintain the heterogeneity of the patient tumor. Recent advancements in in vitro 3-D culture systems such as primary patient-derived organoids can meet this need by providing novel cancer models that better mimic the microenvironment of the originating tumor and exhibit a stable phenotype. A key feature of organoid culture involves embedding cells within a nondefined extracellular matrix that permits the cells to grow in three dimensions into large, complex structures with varying morphologies. However, these features can also make routine quantification of culture health and proliferation challenging. Unlike simpler 2-D monolayer cell cultures, organoids do not proliferate as single cells, which can make cell counting and viability quantification approaches difficult. The extracellular matrix in which organoids are embedded may also require removal, which necessitates additional hands-on processing. Additionally, growth in 3-D can interfere with simple visual assessment via brightfield or phase contrast imaging. Here we applied several common approaches for quantification of cell culture health and proliferation to primary patient-derived cancer organoids from multiple tissues, donors, and cancer types. Approaches including commercially available kits to quantify metabolism or ATP and the common trypan blue dye exclusion assay were utilized. The results were compared with label-free imaging approaches from multiple instrument platforms, which assess growth based on morphological features in brightfield or phase contrast images over time. Additionally, a small-scale toxicity assay was performed with various chemotherapy drugs to assess the discrimination ability of the assays. Results varied between models, donors, tissues, and cancer types. All methods were able to capture long-term changes in organoid proliferation, though all faced unique challenges, typically around sample preparation. Traditional in vitro assays designed for 2-D monolayer cultures could be impeded by the presence of extracellular matrix and had difficulty in penetrating large, multicellular organoids. This resulted in lower signals or higher backgrounds, unless the samples were preprocessed. Imaging-based approaches required significant customization and optimization on a per-model basis. Overall, we found that to accurately assess the growth properties of such complex three-dimensional organoid cultures, significant optimization and validation may be required. Depending on the specific application, either imaging based or cell-based assay approaches may be suitable.
Download the poster to discover how to assess the growth properties of organoid cultures.
Download